are endergonic reactions spontaneous
Web The only way that an endergonic reaction can occur spontaneously is if it is coupled with an even more exergonic reaction. These reactions occur spontaneously.
 |
| Ppt Endergonic And Exergonic Reactions Powerpoint Presentation Free Download Id 1310676 |
Spontaneous reactions are also defined in the same way as far as I know.

. Web The only way that an endergonic reaction can occur spontaneously is if it is coupled with an even more exergonic reaction. This pattern is linked to stability. Web These reactions release energy in its surroundings and there is a. Khan Academy is a nonprofit with the.
Web In a spontaneous process every reactant tends to generate the corresponding product. For this reaction endergonic and. The progress of the reaction is shown by the line. Web An exergonic reaction is a reaction that releases free energy.
An endergonic reaction will not take place on its own without the. Because this type of reaction releases energy rather than consuming it it can occur spontaneously. The sum of the ΔG values of the. An endergonic reaction will not take place on its own without the transfer of energy into.
Web Study with Quizlet and memorize flashcards containing terms like Select all of the following statements that correctly describe endergonic reactions. Web These chemical reactions are called endergonic reactions and they are non-spontaneous. Endergonic reactions are non-spontaneous meaning that energy must be added before they can proceed. Web Learn for free about math art computer programming economics physics chemistry biology medicine finance history and more.
Web Forward and Reverse Reactions. The sum of the ΔG values of the two reactions is then negative. If a reaction is endergonic in one direction it is exergonic in the other direction and vice versa. Web The only way that an endergonic reaction can occur spontaneously is if it is coupled with an even more exergonic reaction.
The change of Gibbs. The sum of the ΔG values of the two. All physical and chemical systems in the universe follow the second law of thermodynamics and proceed in a downhill ie exergonic direction. Web Exergonic reactions have a negative Δ G.
Web Are all endergonic reactions Nonspontaneous. - An input of energy is required. The overall reaction becomes exergonic and spontaneous. Web This information is very valuable because such spontaneous reactions can be tapped to do useful work such as in metabolism.
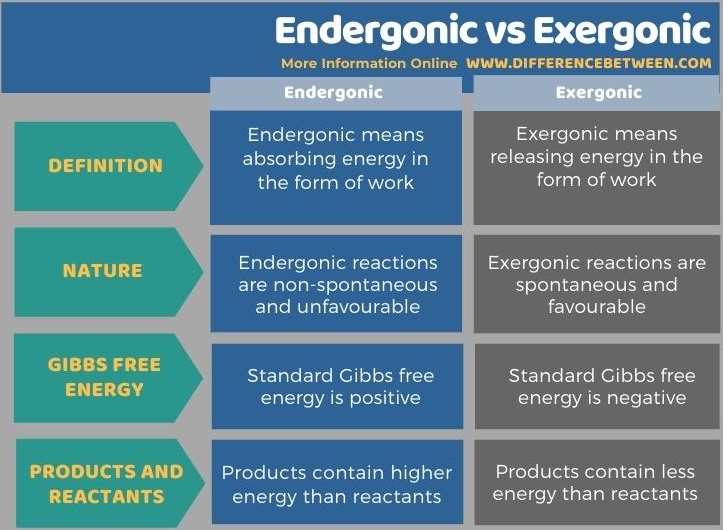
Web Web Spontaneous reactions are exergonic but non-spontaneous reactions are endergonic. Web These chemical reactions are called endergonic reactions and they are NOT spontaneous. Web Do endergonic reactions occur spontaneously. Web Endergonic means absorbing energy in the form of work Endergonic reactions are not spontaneous.
However enzymes do speed up the rate of a spontaneous reaction. Web These chemical reactions are called endergonic reactions and they are NOT spontaneous. Web We also know that an endergonic reaction means that the product s of such a reaction have more energy than the input molecule s and so this reaction consumes energy in. If the ΔG 0.
The system loses free energy. An endergonic reaction will not take place on its own without transferring. An exergonic reaction is a chemical reaction where. Endergonic reactions are nonspontaneous.
A chemical reaction known as an. Thus left to itself any physical or chemical system will proceed according to the second law of thermodynamics in a direction that tends to lower the free energy of the system and thus to expend energy in the form of work. Web Now enzymes do NOT make a non-spontaneous reaction spontaneous.
 |
| Endergonic Exergonic Exothermic And Endothermic Video Khan Academy |
 |
| Structural Biochemistry Endergonic Reaction Wikibooks Open Books For An Open World |
 |
| Difference Between Endergonic And Exergonic Compare The Difference Between Similar Terms |
 |
| Week 4 Energy And Enzymes Ppt Download |
 |
| Endergonic Reaction Wikipedia |
Posting Komentar untuk "are endergonic reactions spontaneous"